
Breaking News
 Battleborn Batteries Responds! Their Overheating Device is a "Feature" not a "Problem
Battleborn Batteries Responds! Their Overheating Device is a "Feature" not a "Problem
 Actor Liam Neeson Outs Himself as MAHA After Narrating Pro-RFK Jr. Documentary Slamming...
Actor Liam Neeson Outs Himself as MAHA After Narrating Pro-RFK Jr. Documentary Slamming...
 Kyle Rittenhouse announced on social media Wednesday that he has tied the knot.
Kyle Rittenhouse announced on social media Wednesday that he has tied the knot.
 JUST IN: President Trump Grants Tina Peters Pardon
JUST IN: President Trump Grants Tina Peters Pardon
Top Tech News
 Build a Greenhouse HEATER that Lasts 10-15 DAYS!
Build a Greenhouse HEATER that Lasts 10-15 DAYS!
 Look at the genius idea he came up with using this tank that nobody wanted
Look at the genius idea he came up with using this tank that nobody wanted
 Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
 Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
 Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
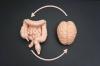 We've wiretapped the gut-brain hotline to decode signals driving disease
We've wiretapped the gut-brain hotline to decode signals driving disease
 3D-printable concrete alternative hardens in three days, not four weeks
3D-printable concrete alternative hardens in three days, not four weeks
 Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
GSK agrees to settle about 80,000 Zantac lawsuits for up to $2.2 bln

The agreement with 10 plaintiffs' law firms resolves about 80,000 cases, or 93% of cases pending against the British drugmaker in state courts nationwide, the company said. GSK also said it would pay $70 million to settle a related whistleblower lawsuit filed by a Connecticut laboratory.
GSK did not admit wrongdoing as part of the deal, saying in a statement that there was "no consistent or reliable evidence" that ranitidine, the drug's active ingredient, increased the risk of cancer. However, it said the settlements were in the best long-term interest of the company to avoid the risk of continuing litigation.
Jennifer Moore and R. Brent Wisner, lead attorneys for the plaintiffs, said in a joint statement that they were "thrilled" with the deal.
First approved by U.S. regulators in 1983, Zantac became the world's best-selling medicine in 1988 and one of the first to top $1 billion in annual sales. The drug was sold at different times by pharmaceutical companies GSK, Pfizer (PFE.N), opens new tab, Sanofi (SASY.PA), opens new tab and Boehringer Ingelheim.
Lawsuits against the companies began piling up in both state and federal courts after the U.S. Food and Drug Administration in 2020 asked manufacturers to pull Zantac off the market. The agency cited concerns that ranitidine could degrade into NDMA, a carcinogen, over time or when exposed to heat.
Pfizer has agreed to settle most of the Zantac cases against it in state court, according to its most recent financial statement, and Sanofi in April announced that it was settling about 4,000 cases.
Boehringer Ingelheim has not announced any major settlements, but is currently facing a trial over the drug in Oakland, California, state court. The company has denied wrongdoing.
"We continue to pursue claims against Boehringer Ingelheim for its wrongdoing for exposing millions of people to a known carcinogen for over a decade," Moore and Wisner said.

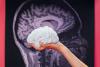 First totally synthetic human brain model has been realized
First totally synthetic human brain model has been realized Mach-23 potato gun to shoot satellites into space
Mach-23 potato gun to shoot satellites into space

