
Breaking News
 Team USA Freestyle Skiers Express 'Mixed Emotions' and 'Heartbreak'...
Team USA Freestyle Skiers Express 'Mixed Emotions' and 'Heartbreak'...
 The Safety Cult is Going to Kill Us All
The Safety Cult is Going to Kill Us All
 10 Things I Learned From the Epstein Files
10 Things I Learned From the Epstein Files
 He's EXPOSING the truth about SSRI's and Anti-Depressants, and they are P*SSED
He's EXPOSING the truth about SSRI's and Anti-Depressants, and they are P*SSED
Top Tech News
 How underwater 3D printing could soon transform maritime construction
How underwater 3D printing could soon transform maritime construction
 Smart soldering iron packs a camera to show you what you're doing
Smart soldering iron packs a camera to show you what you're doing
 Look, no hands: Flying umbrella follows user through the rain
Look, no hands: Flying umbrella follows user through the rain
 Critical Linux Warning: 800,000 Devices Are EXPOSED
Critical Linux Warning: 800,000 Devices Are EXPOSED
 'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
 The smartphone just fired a warning shot at the camera industry.
The smartphone just fired a warning shot at the camera industry.
 A revolutionary breakthrough in dental science is changing how we fight tooth decay
A revolutionary breakthrough in dental science is changing how we fight tooth decay
 Docan Energy "Panda": 32kWh for $2,530!
Docan Energy "Panda": 32kWh for $2,530!
 Rugged phone with multi-day battery life doubles as a 1080p projector
Rugged phone with multi-day battery life doubles as a 1080p projector
 4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
AstraZeneca Coronavirus Vaccine to Be Pulled Worldwide for 'Commercial' Reasons
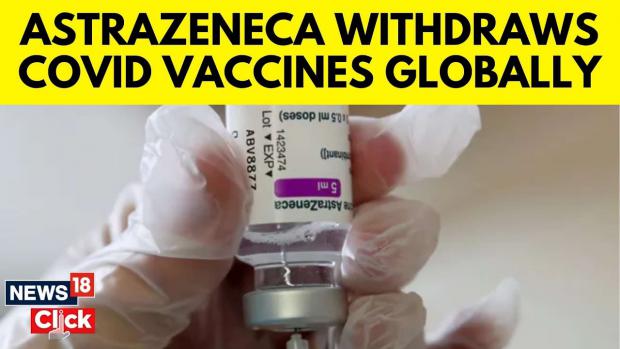
The Oxford-AstraZeneca coronavirus vaccine will be withdrawn worldwide just months after the firm admitted that the jab causes a rare but dangerous side effect, however, AstraZeneca insists that the decision to pull the vaccine was purely made for commercial reasons.
AstraZeneca pulled its "marketing authorisation" for its COVID-19 vaccine, known commercially as Vaxzevria, in the European Union on Tuesday and plans to do so in the UK and other countries where it was deployed in the coming months. The decision will not impact the United States, where the jab never received approval to go to market.
The pharmaceutical giant insisted that the decision was made for commercial considerations, saying that the vaccine is no longer being manufactured and that more recent vaccines have been developed to confront novel variants of the Chinese virus.



