
Breaking News
 One company does over 50% of all school photos in America and over 25% of school photos globally
One company does over 50% of all school photos in America and over 25% of school photos globally
 Your Water Filter Will Clog - The Medieval Sand Filtration System That Purifies Forever
Your Water Filter Will Clog - The Medieval Sand Filtration System That Purifies Forever
 Aaron Day - BTC and Stable Coins: 'The Creature From Epstein Island' (Publisher Recommended)
Aaron Day - BTC and Stable Coins: 'The Creature From Epstein Island' (Publisher Recommended)
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
US firm wins FDA approval for groundbreaking clinical trial that will see implants placed...
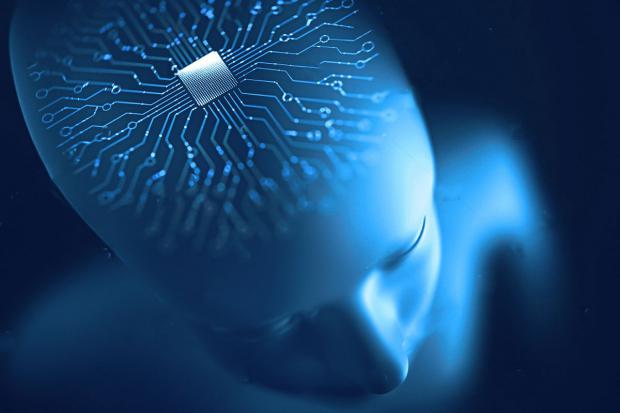
Elon Musk might be well positioned in space travel and electric vehicles, but the world's second-richest person is taking a backseat when it comes to a brain-computer interface (BCI).
New York-based Synchron announced Wednesday that it has received approval from the Food and Drug Administration to begin clinical trials of its Stentrode motor neuroprosthesis - a brain implant it is hoped could ultimately be used to cure paralysis.
The FDA approved Synchron's Investigational Device Exemption (IDE) application, according to a release, paving the way for an early feasibility study of Stentrode to begin later this year at New York's Mount Sinai Hospital.
The study will analyze the safety and efficacy of the device, smaller than a matchstick, in six patients with severe paralysis.
Meanwhile, Musk has been touting Neuralink, his brain-implant startup, for several years—most recently showing a video of a monkey with the chip playing Pong using only signals from its brain.
However, the company reportedly has been plagued by setbacks and unrealistic timelines.
'The approval of this IDE reflects years of safety testing performed in conjunction with FDA,' Synchron CEO Thomas Oxley said in the release.
'We have worked together to pave a pathway forward, towards the first commercial approval for a permanently implanted BCI for the treatment of paralysis. We are thrilled to finally be launching a U.S. clinical trial this year.'
Like other BCIs, the Stentrode is intended to provide more independence to those with brain injuries, trauma, ALS or other conditions that sever the connections between the brain and the body's motor control system.

 Why We'll Win
Why We'll Win
 Smart dust technology...
Smart dust technology...

