
Breaking News
 $11 Trillion Quietly Moved - Americans Will Freeze & Obey When Market Collapse Hits : Chase Hughes
$11 Trillion Quietly Moved - Americans Will Freeze & Obey When Market Collapse Hits : Chase Hughes
 Econ 101 - 2026 Early Economic Forecast
Econ 101 - 2026 Early Economic Forecast
 Buy'r breaks the Blackrock monopoly- How the corporate club controls America
Buy'r breaks the Blackrock monopoly- How the corporate club controls America
 An AI Expert Warning: 6 People Are (Quietly) Deciding Humanity's Future! We Must Act Now!
An AI Expert Warning: 6 People Are (Quietly) Deciding Humanity's Future! We Must Act Now!
Top Tech News
 Build a Greenhouse HEATER that Lasts 10-15 DAYS!
Build a Greenhouse HEATER that Lasts 10-15 DAYS!
 Look at the genius idea he came up with using this tank that nobody wanted
Look at the genius idea he came up with using this tank that nobody wanted
 Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
Latest Comet 3I Atlas Anomolies Like the Impossible 600,000 Mile Long Sunward Tail
 Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
Tesla Just Opened Its Biggest Supercharger Station Ever--And It's Powered By Solar And Batteries
 Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
Your body already knows how to regrow limbs. We just haven't figured out how to turn it on yet.
 We've wiretapped the gut-brain hotline to decode signals driving disease
We've wiretapped the gut-brain hotline to decode signals driving disease
 3D-printable concrete alternative hardens in three days, not four weeks
3D-printable concrete alternative hardens in three days, not four weeks
 Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
Could satellite-beaming planes and airships make SpaceX's Starlink obsolete?
DARPA Nucleic Technology Funded to Get Virus Response to 1-60 Days
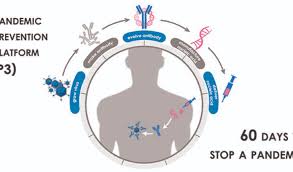
The P3 teams are developing technologies to deliver antibodies using nucleic acid technology, and achieve sufficient serum concentrations of the antibodies for protection against the pathogen within three days after administration.
DARPA has programs for Pandemic Prevention Platform, Nucleic Acids On-Demand Worldwide (NOW), and PReemptive Expression of Protective Alleles and Response Elements (PREPARE) programs.
Nucleic Acids On-Demand Worldwide (NOW)
The goal of the NOW program is to develop a mobile medical countermeasure (MCM) manufacturing platform for use in stabilization and humanitarian operations to produce, formulate, and package hundreds of doses of nucleic acid therapeutics (DNA and/or RNA) in less than 24 hours. The developed platform should be a resilient, mobile, readily accessible nucleic acid MCM manufacturing capability that enables immediate threat response anywhere the military operates with minimal user intervention.

 First totally synthetic human brain model has been realized
First totally synthetic human brain model has been realized Mach-23 potato gun to shoot satellites into space
Mach-23 potato gun to shoot satellites into space

