
Breaking News
 Boston Dynamics' Atlas just did a roundoff back handspring… yeah we're cooked
Boston Dynamics' Atlas just did a roundoff back handspring… yeah we're cooked
 It's Not Just Pakistan - Foreigners from Around the World Who Are Not US Citizens...
It's Not Just Pakistan - Foreigners from Around the World Who Are Not US Citizens...
 Elon Musk's Darkest Secret - Dr. Eric Weinstein
Elon Musk's Darkest Secret - Dr. Eric Weinstein
 Puerto Rico's Rep. Rivera Turned The Halftime Show Into an Anti-ICE Rant (VIDEO)
Puerto Rico's Rep. Rivera Turned The Halftime Show Into an Anti-ICE Rant (VIDEO)
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Progress to Practical Room Temperature Superconductors
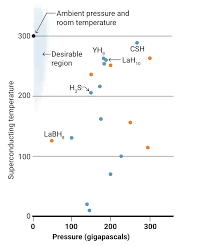
Theorists have predicted other hydride compounds which could work at lower pressures. There is race to find versions stable at ambient pressure and room temperature.
In 2004, Ashcroft suggested that adding other elements to hydrogen might add a "chemical precompression," stabilizing the hydrogen lattice at lower pressures. The race was on to make superconducting hydrides. In 2015, researchers including Mikhail Eremets, a physicist at the Max Planck Institute for Chemistry, reported in Nature that a mix of sulfur and hydrogen superconducted at 203 K when pressurized to 155 GPa. Over the next 3 years, Eremets and others boosted the Tc as high as 250 K in hydrides containing the heavy metal lanthanum. Then came Dias's CSH compound, reported late last year in Nature, which superconducts at 287 K—or 14°C, the temperature of a wine cellar—under 267 GPa of pressure, followed by an yttrium hydride that superconducts at nearly as warm a temperature, announced by multiple groups this year.

 Smart dust technology...
Smart dust technology...

