
Breaking News
 Ultimate House of Cards: $5.1 Trillion Bond Fraud Set to Dwarf 2008 Crisis
Ultimate House of Cards: $5.1 Trillion Bond Fraud Set to Dwarf 2008 Crisis
 Escalation of Force: How to Choose the Appropriate Response to Potential Violence
Escalation of Force: How to Choose the Appropriate Response to Potential Violence
 Epstein's Island And The Gateway To The Psychology Of Evil
Epstein's Island And The Gateway To The Psychology Of Evil
 The Epstein Emails Reveal Shadow 9/11 Commission – Exclusive Report!
The Epstein Emails Reveal Shadow 9/11 Commission – Exclusive Report!
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Artificial Intelligence creating new drugs from scratch by efficiently searching...
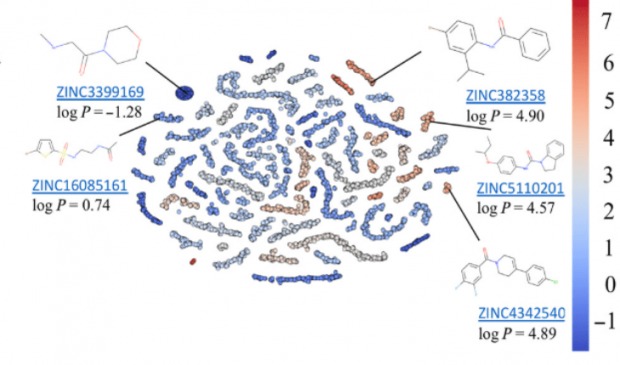
AI is revolutionizing medicine including radiology, pathology, and other medical specialties. Deep learning (DL) technologies are beginning to find applications in drug discovery including areas of molecular docking, transcriptomics, reaction mechanism elucidation, and molecular energy prediction.
A crucial step in many new drug discovery projects is the formulation of a well-motivated hypothesis for new lead compound generation (de novo design) or compound selection from available or synthetically feasible chemical libraries based on the available structure-activity relationship (SAR) data. The design hypotheses are often biased toward preferred chemistry or driven by model interpretation. Automated approaches for designing compounds with the desired properties de novo have become an active field of research in the last 15 years. The diversity of synthetically feasible chemicals that can be considered as potential drug-like molecules was estimated to be between 1030 and 1060. Great advances in computational algorithms, hardware, and high-throughput screening technologies notwithstanding, the size of this virtual library prohibits its exhaustive sampling and testing by systematic construction and evaluation of each individual compound. Local optimization approaches have been proposed, but they do not ensure the optimal solution, as the design process converges on a local or "practical" optimum by stochastic sampling or restricts the search to a defined section of chemical space that can be screened exhaustively.
Researchers have designed and implemented a novel computational strategy for de novo design of molecules with desired properties termed ReLeaSE (Reinforcement Learning for Structural Evolution). On the basis of deep and reinforcement learning (RL) approaches, ReLeaSE integrates two deep neural networks—generative and predictive—that are trained separately but are used jointly to generate novel targeted chemical libraries. ReLeaSE uses simple representation of molecules by their simplified molecular-input line-entry system (SMILES) strings only. Generative models are trained with a stack-augmented memory network to produce chemically feasible SMILES strings, and predictive models are derived to forecast the desired properties of the de novo–generated compounds. In the first phase of the method, generative and predictive models are trained separately with a supervised learning algorithm. In the second phase, both models are trained jointly with the RL approach to bias the generation of new chemical structures toward those with the desired physical and/or biological properties. In the proof-of-concept study, we have used the ReLeaSE method to design chemical libraries with a bias toward structural complexity or toward compounds with maximal, minimal, or specific range of physical properties, such as melting point or hydrophobicity, or toward compounds with inhibitory activity against Janus protein kinase 2. The approach proposed herein can find a general use for generating targeted chemical libraries of novel compounds optimized for either a single desired property or multiple properties.

 Smart dust technology...
Smart dust technology...

