
Breaking News
 Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
 Elon Musk Offers To Cover Legal Bills Of Epstein Survivors Who Identify New Names
Elon Musk Offers To Cover Legal Bills Of Epstein Survivors Who Identify New Names
 Red Alert Emergency Broadcast! Tune In NOW As Alex Jones Analyzes The Insane Revelations...
Red Alert Emergency Broadcast! Tune In NOW As Alex Jones Analyzes The Insane Revelations...
 330 gallons of sulphuric acid was purchased for Epstein Island on the day the FBI opened...
330 gallons of sulphuric acid was purchased for Epstein Island on the day the FBI opened...
Top Tech News
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
A Brilliant New Cancer Treatment That Re-Engineers Human Cells Just Got Approved
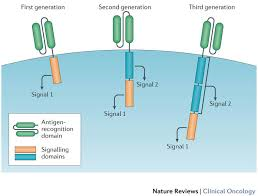
The US Food and Drug Administration (FDA) just approved a cutting-edge cancer therapy.
On Wednesday, the FDA approved Novartis's Kymriah, also known as tisagenlecleucel, a treatment for pediatric acute lymphoblastic lymphoblastic leukemia.
"I think this is most exciting thing I've seen in my lifetime," Dr. Tim Cripe, an oncologist who was part of the FDA advisory committee panel that voted in favour of approving the drug in July.
The highly personalised treatment is called CAR T-cell therapy. It's a type of cancer immunotherapy — or a therapy that harnesses the body's immune system to take on cancer cells.
"We're entering a new frontier in medical innovation with the ability to reprogram a patient's own cells to attack a deadly cancer," FDA commissioner Scott Gottlieb said in a statement.



