
Breaking News
 Powerful Pro-life Ad Set to Air During Super Bowl 'Adoption is an Option' (Video)
Powerful Pro-life Ad Set to Air During Super Bowl 'Adoption is an Option' (Video)
 Even in Winter, the Sun Still Shines in These Citrus Recipes
Even in Winter, the Sun Still Shines in These Citrus Recipes
 Dates: The Ancient Fertility Remedy Modern Medicine Ignores Amid Record Low Birth Rates
Dates: The Ancient Fertility Remedy Modern Medicine Ignores Amid Record Low Birth Rates
 Amazon's $200 Billion Spending Shock Reveals Big Tech's Centralization Crisis
Amazon's $200 Billion Spending Shock Reveals Big Tech's Centralization Crisis
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Resetting Clock on Aging Cells Safely Reversed Signs of Decline in Mice
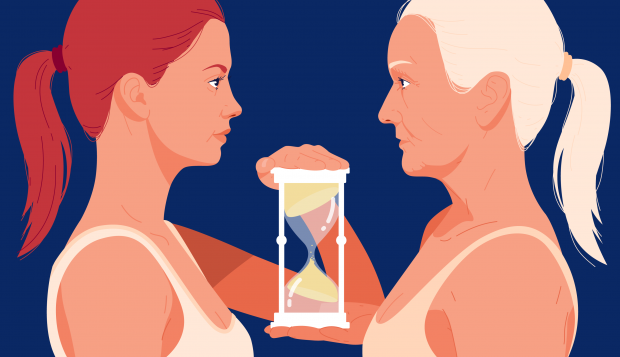
Now, scientists at the Salk Institute have shown that they can reverse the aging process in middle-aged and elderly mice, leading to a variety of benefits.
The technique works by partially resetting their cells to more youthful states, which impact skin, eyesight, muscles, and the brain.
"We are elated that we can use this approach across the life span to slow down aging in normal animals. The technique is both safe and effective in mice," says co-corresponding author Juan Carlos Izpisua Belmonte, professor in Salk's Gene Expression Laboratory.
When injured, the youthful skin of the treated mice had a greater ability to heal and was less likely to form permanent scars.
Both the kidneys and blood of treated animals more closely resembled epigenetic patterns seen in younger animals.
As organisms age, it is not just their outward appearances and health that change; every cell in their bodies carries a molecular clock that records the passage of time. Cells isolated from older people or animals have different patterns of chemicals along their DNA—called epigenetic markers. Scientists know that adding a mixture of four reprogramming molecules—Oct4, Sox2, Klf4 and cMyc, also known as 'Yamanaka factors'—to cells can reset these epigenetic marks to their original patterns.
Scientists have used this approach in experiments to improve the function of other tissues like the heart, brain, and eyesight.
At Salk, they tested three groups of mice at varying ages equivalent to humans being 35, 50 and age 80, and found after seven or 10 months, the mice resembled younger animals in both appearance and ability.

 Smart dust technology...
Smart dust technology...

