
Breaking News
 Trump has pros as well as cons
Trump has pros as well as cons
 Congress To Have Access to Unredacted Epstein Files
Congress To Have Access to Unredacted Epstein Files
 How $30BILLION of taxpayers' money put aside for welfare pot became a 'slush fund'...
How $30BILLION of taxpayers' money put aside for welfare pot became a 'slush fund'...
 Why This Crash Is Bitcoin's Biggest Test Yet
Why This Crash Is Bitcoin's Biggest Test Yet
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
39-Year-Old Becomes First US Patient to Receive Innovative Artificial Heart Prosthetic
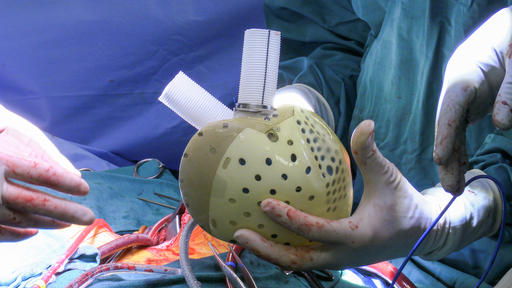
The artificial heart was developed by a French company, CARMAT and has been approved for use and sale in Europe.
Last year, the company received U.S. FDA approval to begin studies and enroll 10 patients with end-stage biventricular heart failure—people who are suffering on the waiting list for a heart donor—and offer a life-saving bridge before transplant.
"We are encouraged that our patient is doing so well after the procedure Monday," said Dr. Carmelo Milano, a transplant surgeon and the principal investigator of the device study at Duke. "As we evaluate this device, we are both excited and hopeful that patients who otherwise have few to no options could have a lifeline."
The North Carolina patient, Matthew Moore, is just 39-years-old and was referred to Duke in June after a sudden, unexpected diagnosis of heart failure. Moore and his wife, Rachel, recently adopted their two-year-old foster son, Marshall, and arrived at Duke expecting only to undergo heart bypass surgery.

 Smart dust technology...
Smart dust technology...

