
Breaking News
 One company does over 50% of all school photos in America and over 25% of school photos globally
One company does over 50% of all school photos in America and over 25% of school photos globally
 Your Water Filter Will Clog - The Medieval Sand Filtration System That Purifies Forever
Your Water Filter Will Clog - The Medieval Sand Filtration System That Purifies Forever
 Aaron Day - BTC and Stable Coins: 'The Creature From Epstein Island' (Publisher Recommended)
Aaron Day - BTC and Stable Coins: 'The Creature From Epstein Island' (Publisher Recommended)
Top Tech News
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
 Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
Study Shows Vaporizing E-Waste Makes it Easy to Recover Precious Metals at 13-Times Lower Costs
New evidence strengthens link between telomere length, aging and cancer
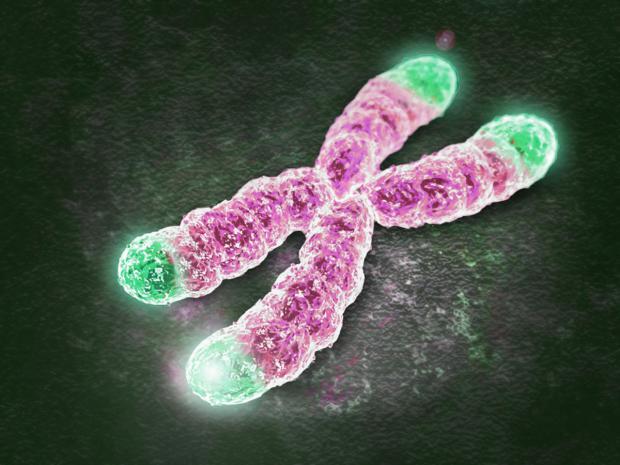
Now a new study has found an intriguing piece of evidence supporting this hypothesis in genomes from several families that seem to be particularly prone to cancer.
In a way, our cells have a pre-determined number of divisions in their lifetime – around 50. That limit is dictated by our telomeres, small repeating segments of "junk" DNA that form caps on the ends of our chromosomes. These act like a buffer protecting the important DNA in the chromosomes from damage when a cell divides, but a little piece of the telomere is lost each time.
Eventually that damage adds up and the telomeres shorten to the point that the cell stops dividing. This contributes to the symptoms of aging that we're all too familiar with.
In theory, lengthening our telomeres or preventing them from shrinking should help slow the aging process, or even reverse it. Indeed, plenty of research is investigating this angle. But there's a nasty potential downside to doing so – cancer.
Cancer cells are effectively immortal, in the sense that they never stop dividing. It appears that having telomeres of a set length is an evolutionary defense mechanism to prevent that kind of runaway growth. And the new study has found more evidence supporting that hypothesis.
Researchers at the Rockefeller University and the Radboud University Medical Center studied the genomes of several Dutch families that appeared to be quite cancer-prone. Common among these patients were mutations in a gene called TINF2, which codes for a protein previously linked to telomere length.
So the team used CRISPR to engineer human cells with the same mutations, and found that they had much longer telomeres than usual. When the scientists checked the patients themselves, these little caps were also found to be particularly long.

 Why We'll Win
Why We'll Win
 Smart dust technology...
Smart dust technology...

