
Breaking News
 Palantir kills people? But Who's Really Pushing the Buttons?
Palantir kills people? But Who's Really Pushing the Buttons?
 'Big Short' investor Michael Burry sounds alarm on AI bubble that's 'too big to save
'Big Short' investor Michael Burry sounds alarm on AI bubble that's 'too big to save
 2026-01-21 -- Ernest Hancock interviews Professor James Corbett (Corbett Report) MP3&4
2026-01-21 -- Ernest Hancock interviews Professor James Corbett (Corbett Report) MP3&4
 Joe rogan reacts to the Godfather of Ai Geoffrey Hinton talk of his creation
Joe rogan reacts to the Godfather of Ai Geoffrey Hinton talk of his creation
Top Tech News
 The day of the tactical laser weapon arrives
The day of the tactical laser weapon arrives
 'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
 Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
 XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
 Watch: World's fastest drone hits 408 mph to reclaim speed record
Watch: World's fastest drone hits 408 mph to reclaim speed record
 Ukrainian robot soldier holds off Russian forces by itself in six-week battle
Ukrainian robot soldier holds off Russian forces by itself in six-week battle
 NASA announces strongest evidence yet for ancient life on Mars
NASA announces strongest evidence yet for ancient life on Mars
 Caltech has successfully demonstrated wireless energy transfer...
Caltech has successfully demonstrated wireless energy transfer...
 The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
Breakthrough for rechargeable non-aqueous magnesium-metal battery...
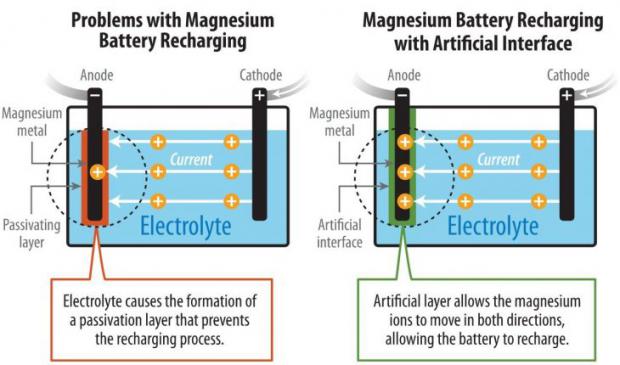
Scientists have pioneered a method to enable the reversible chemistry of magnesium metal in the noncorrosive carbonate-based electrolytes and tested the concept in a prototype cell. The technology possesses potential advantages over lithium-ion batteries—notably, higher energy density, greater stability, and lower cost.
Magnesium (Mg) batteries theoretically contain almost twice as much energy per volume as lithium-ion batteries. But previous research encountered an obstacle: chemical reactions of the conventional carbonate electrolyte created a barrier on the surface of magnesium that prevented the battery from recharging. The magnesium ions could flow in a reverse direction through a highly corrosive liquid electrolyte, but that barred the possibility of a successful high-voltage magnesium battery.

 Nano Nuclear Enters The Asian Market
Nano Nuclear Enters The Asian Market


