
Breaking News
 Tuesday War Room LIVE: Trump Set to Shatter Deportation Record by End of First Year…
Tuesday War Room LIVE: Trump Set to Shatter Deportation Record by End of First Year…
 Parallel Polis Reborn: Freeing the Market through Decentralized Technologies
Parallel Polis Reborn: Freeing the Market through Decentralized Technologies
 Amazon goes nuclear with new modular reactor plant
Amazon goes nuclear with new modular reactor plant
 The alarming reality EXPOSED by the global internet meltdown... and why Amazon's crash...
The alarming reality EXPOSED by the global internet meltdown... and why Amazon's crash...
Top Tech News
 3D Printed Aluminum Alloy Sets Strength Record on Path to Lighter Aircraft Systems
3D Printed Aluminum Alloy Sets Strength Record on Path to Lighter Aircraft Systems
 Big Brother just got an upgrade.
Big Brother just got an upgrade.
SEMI-NEWS/SEMI-SATIRE: October 12, 2025 Edition
 Stem Cell Breakthrough for People with Parkinson's
Stem Cell Breakthrough for People with Parkinson's
 Linux Will Work For You. Time to Dump Windows 10. And Don't Bother with Windows 11
Linux Will Work For You. Time to Dump Windows 10. And Don't Bother with Windows 11
 XAI Using $18 Billion to Get 300,000 More Nvidia B200 Chips
XAI Using $18 Billion to Get 300,000 More Nvidia B200 Chips
 Immortal Monkeys? Not Quite, But Scientists Just Reversed Aging With 'Super' Stem Cells
Immortal Monkeys? Not Quite, But Scientists Just Reversed Aging With 'Super' Stem Cells
 ICE To Buy Tool That Tracks Locations Of Hundreds Of Millions Of Phones Every Day
ICE To Buy Tool That Tracks Locations Of Hundreds Of Millions Of Phones Every Day
 Yixiang 16kWh Battery For $1,920!? New Design!
Yixiang 16kWh Battery For $1,920!? New Design!
 Find a COMPATIBLE Linux Computer for $200+: Roadmap to Linux. Part 1
Find a COMPATIBLE Linux Computer for $200+: Roadmap to Linux. Part 1
Precise control of the properties of plastics
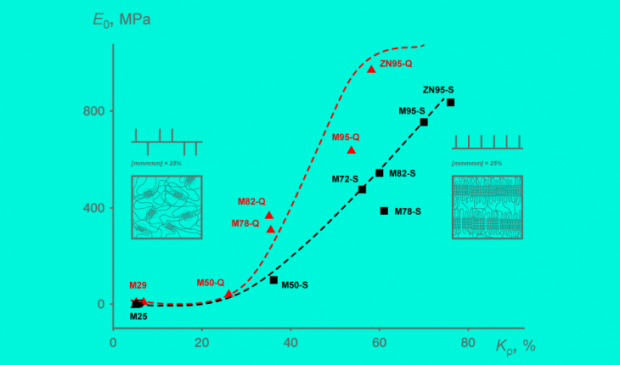
Their new insights make it possible to synthesize a material with predetermined properties, such as elasticity or hardness.
Polypropylene is so ubiquitous one might call it the king of plastics. In terms of production volume, it is second only to polyethylene. By tweaking its molecular structure, polypropylene can be used to manufacture materials with a wide range of features, from elastic bands to high-impact plastic. However, the relationship between the polymer's chemical structure and its mechanical properties was not fully understood.
A polypropylene chain consists of a backbone of carbon atoms with attached hydrogen atoms. Every other carbon atom in the chain has a methyl group attached to it. Two adjacent carbon atoms in the chain with the hydrogen atoms and the methyl group bonded to them constitute a repeating unit called propylene, or propene. The spatial configuration of the macromolecule — the polymer chain — is determined by the mutual orientation of the methyl groups in the chain : If they are all on one side, the molecule is said to be isotactic.



