
Breaking News
 The Pentagon Failed Its Audit Again. You Should Be Alarmed.
The Pentagon Failed Its Audit Again. You Should Be Alarmed.
 Cuban Crisis 2.0. What if 'Gerans' flew from Cuba?
Cuban Crisis 2.0. What if 'Gerans' flew from Cuba?
 Senate Democrats Offer Promising Ideas for Changing Immigration Enforcement
Senate Democrats Offer Promising Ideas for Changing Immigration Enforcement
 Never Seen Risk Like This Before in My Career
Never Seen Risk Like This Before in My Career
Top Tech News
 Critical Linux Warning: 800,000 Devices Are EXPOSED
Critical Linux Warning: 800,000 Devices Are EXPOSED
 'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
 The smartphone just fired a warning shot at the camera industry.
The smartphone just fired a warning shot at the camera industry.
 A revolutionary breakthrough in dental science is changing how we fight tooth decay
A revolutionary breakthrough in dental science is changing how we fight tooth decay
 Docan Energy "Panda": 32kWh for $2,530!
Docan Energy "Panda": 32kWh for $2,530!
 Rugged phone with multi-day battery life doubles as a 1080p projector
Rugged phone with multi-day battery life doubles as a 1080p projector
 4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
 Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
 New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
 Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
A Novel Aluminum–Graphite Dual-Ion Battery
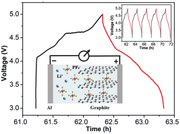
A packaged aluminum–graphite battery is estimated to deliver an energy density of ≈150 Wh kg−1 at a power density of ≈1200 W kg−1, which is ≈50% higher than most commercial lithium ion batteries.
Lithium ion batteries based on cation intercalation have been powering the increasingly mobile society for decades.[1] In a conventional lithium ion battery, the intercalation of lithium ions in both cathode (i.e., LiCoO2, LiFePO4) and anode (i.e., graphite, silicon) materials have been thoroughly studied, while the utilization of the anions in the electrolyte has drawn much less attention.[2] In fact, the phenomenon of anion intercalate into graphite by chemical or electrochemical means was discovered and proposed as a possible positive electrode for batteries by Rüdorff and Hofmann in 1938.[3] However, the anion intercalation was achieved by using high concentration acid solution as electrolyte, this brought serious safety issue that hindered its application.[4] In the 1990s, soon after the commercial application of lithium ion battery, Carlin et al. reported dual graphite intercalating molten electrolyte batteries that realized the application of anion intercalated graphite as positive electrode in batteries by using room temperature ionic liquids as electrolyte.[5] In the following decades, continuous progresses have been made in anion intercalated graphite based dual carbon batteries, such as investigation of anion intercalation in non-aqueous electrolyte, in situ characterization of the staged anion intercalation process, and systematic study of the intercalation of different anions into graphite.[6]



