
Breaking News
 RAY DALIO SAYS THE MONETARY ORDER IS BREAKING DOWN...
RAY DALIO SAYS THE MONETARY ORDER IS BREAKING DOWN...
 2026 - The Year US Hegemony Ends?
2026 - The Year US Hegemony Ends?
 Censorship Lawsuit Big Tech Hoped Wouldn't Happen
Censorship Lawsuit Big Tech Hoped Wouldn't Happen
 House Oversight Panel votes to advance contempt resolutions against the Clintons
House Oversight Panel votes to advance contempt resolutions against the Clintons
Top Tech News
 The day of the tactical laser weapon arrives
The day of the tactical laser weapon arrives
 'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
 Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
 XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
 Watch: World's fastest drone hits 408 mph to reclaim speed record
Watch: World's fastest drone hits 408 mph to reclaim speed record
 Ukrainian robot soldier holds off Russian forces by itself in six-week battle
Ukrainian robot soldier holds off Russian forces by itself in six-week battle
 NASA announces strongest evidence yet for ancient life on Mars
NASA announces strongest evidence yet for ancient life on Mars
 Caltech has successfully demonstrated wireless energy transfer...
Caltech has successfully demonstrated wireless energy transfer...
 The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
Progress to scalable Molecular Machines
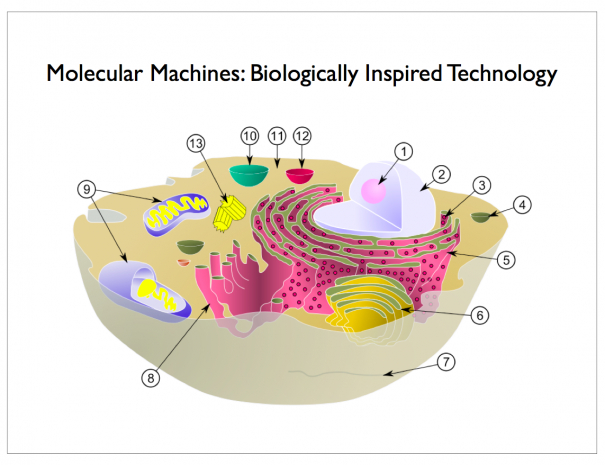
This molecular robot can be programmed to stereoselectively produce, in a sequential one-pot operation, an excess of any one of four possible diastereoisomers from the addition of a thiol and an alkene to an α,β-unsaturated aldehyde in a tandem reaction process. The stereodivergent synthesis includes diastereoisomers that cannot be selectively synthesized through conventional iminium–enamine organocatalysis. They anticipate that future generations of programmable molecular machines may have significant roles in chemical synthesis and molecular manufacturing.
Depending on which side the substrate is held for each reaction, a different stereoisomer of the product is formed. The reaction sequences are carried out in one pot and the robot can be programmed to produce selectively each isomer of the product by controlling the switch-state prior to each reaction of the substrate. Each molecular robot manipulates a single substrate molecule, but the process is massively paralleled with more than a million trillion molecular robots operated simultaneously by the scientists.

 Nano Nuclear Enters The Asian Market
Nano Nuclear Enters The Asian Market


