
Breaking News
 RAY DALIO SAYS THE MONETARY ORDER IS BREAKING DOWN...
RAY DALIO SAYS THE MONETARY ORDER IS BREAKING DOWN...
 2026 - The Year US Hegemony Ends?
2026 - The Year US Hegemony Ends?
 Censorship Lawsuit Big Tech Hoped Wouldn't Happen
Censorship Lawsuit Big Tech Hoped Wouldn't Happen
 House Oversight Panel votes to advance contempt resolutions against the Clintons
House Oversight Panel votes to advance contempt resolutions against the Clintons
Top Tech News
 The day of the tactical laser weapon arrives
The day of the tactical laser weapon arrives
 'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
'ELITE': The Palantir App ICE Uses to Find Neighborhoods to Raid
 Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
Solar Just Took a Huge Leap Forward!- CallSun 215 Anti Shade Panel
 XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
XAI Grok 4.20 and OpenAI GPT 5.2 Are Solving Significant Previously Unsolved Math Proofs
 Watch: World's fastest drone hits 408 mph to reclaim speed record
Watch: World's fastest drone hits 408 mph to reclaim speed record
 Ukrainian robot soldier holds off Russian forces by itself in six-week battle
Ukrainian robot soldier holds off Russian forces by itself in six-week battle
 NASA announces strongest evidence yet for ancient life on Mars
NASA announces strongest evidence yet for ancient life on Mars
 Caltech has successfully demonstrated wireless energy transfer...
Caltech has successfully demonstrated wireless energy transfer...
 The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
The TZLA Plasma Files: The Secret Health Sovereignty Tech That Uncle Trump And The CIA Tried To Bury
New metallic glass material created by starving atoms of a nucleus
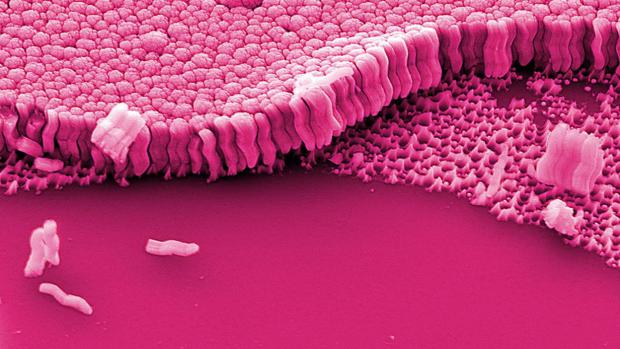
Normally, solid metals have a rigid, crystalline atomic structure, but as their name suggests, metallic glasses are more like glass, with a random arrangement of atoms. Composed of complex alloys, they get their unusual structure when molten metal is cooled down extremely quickly, which prevents crystals from forming. The end result is a material that's as pliable as plastic during production but strong as steel afterwards, making them useful for objects like golf clubs and gears for robots.
The Yale researchers developed their new version of the material by taking samples of metallic glass and making nanorods out of it. With a diameter of just 35 nanometers, these rods are so tiny that the atoms have no room for a nucleus. The researchers dub the process "nucleus starvation," and it resulted in a new phase of the material.
"This gives us a handle to control the number of nuclei we provide in the sample," says Judy Cha, lead researcher on the project. "When it doesn't have any nuclei — despite the fact that nature tells us that there should be one — it generates this brand new crystalline phase that we've never seen before. It's a way to create a new material out of the old."

 Nano Nuclear Enters The Asian Market
Nano Nuclear Enters The Asian Market


