
Breaking News
 Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
 Elon Musk Offers To Cover Legal Bills Of Epstein Survivors Who Identify New Names
Elon Musk Offers To Cover Legal Bills Of Epstein Survivors Who Identify New Names
 Red Alert Emergency Broadcast! Tune In NOW As Alex Jones Analyzes The Insane Revelations...
Red Alert Emergency Broadcast! Tune In NOW As Alex Jones Analyzes The Insane Revelations...
 330 gallons of sulphuric acid was purchased for Epstein Island on the day the FBI opened...
330 gallons of sulphuric acid was purchased for Epstein Island on the day the FBI opened...
Top Tech News
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
Tissue engineering, replacement organs and regenerative medicine are getting friendlier regulations
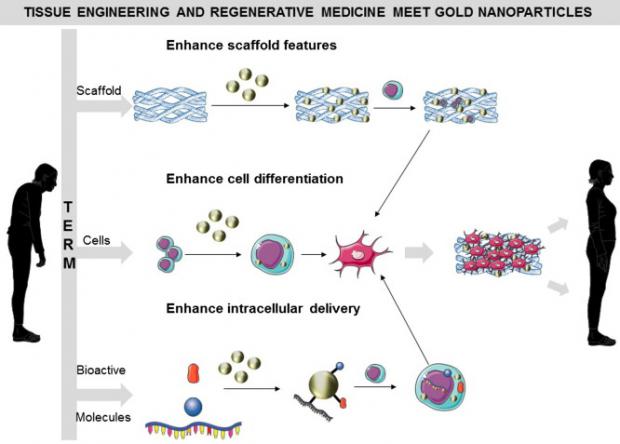
The FDA intends to promote the "least burdensome" rules for companies big and small that are seeking to develop new therapies, "while ensuring patient safety."
"Our policy will allow product manufacturers that time to engage with the FDA to determine if they need to submit a marketing authorization application and, if so, seek guidance on how to submit their application to the FDA for approval," Gottlieb said.
The new rules are in keeping with provisions from the 21st Century Cures Act, passed by Congress in December. That legislation earmarked $6.3 billion in funding, mostly for the U.S. National Institutes of Health, towards groundbreaking medical research.
Over the past few years scientists and physicians have developed tissue-engineered skin for transplant; bladders grown from a patient's own cells; and tissues grown to repair ailing hearts or failing knees.



