
Breaking News
 Grand Theft World Podcast 273 | Goys 'R U.S. with Guest Rob Dew
Grand Theft World Podcast 273 | Goys 'R U.S. with Guest Rob Dew
 Anchorage was the Receipt: Europe is Paying the Price… and Knows it.
Anchorage was the Receipt: Europe is Paying the Price… and Knows it.
 The Slow Epstein Earthquake: The Rupture Between the People and the Elites
The Slow Epstein Earthquake: The Rupture Between the People and the Elites
 Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Israeli Prime Minister, Netanyahu will meet with Trump on Wednesday and deliver instructions...
Top Tech News
 Drone-launching underwater drone hitches a ride on ship and sub hulls
Drone-launching underwater drone hitches a ride on ship and sub hulls
 Humanoid Robots Get "Brains" As Dual-Use Fears Mount
Humanoid Robots Get "Brains" As Dual-Use Fears Mount
 SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
SpaceX Authorized to Increase High Speed Internet Download Speeds 5X Through 2026
 Space AI is the Key to the Technological Singularity
Space AI is the Key to the Technological Singularity
 Velocitor X-1 eVTOL could be beating the traffic in just a year
Velocitor X-1 eVTOL could be beating the traffic in just a year
 Starlink smasher? China claims world's best high-powered microwave weapon
Starlink smasher? China claims world's best high-powered microwave weapon
 Wood scraps turn 'useless' desert sand into concrete
Wood scraps turn 'useless' desert sand into concrete
 Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
Let's Do a Detailed Review of Zorin -- Is This Good for Ex-Windows Users?
 The World's First Sodium-Ion Battery EV Is A Winter Range Monster
The World's First Sodium-Ion Battery EV Is A Winter Range Monster
 China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
China's CATL 5C Battery Breakthrough will Make Most Combustion Engine Vehicles OBSOLETE
FDA quietly bans powerful life-saving intravenous Vitamin C
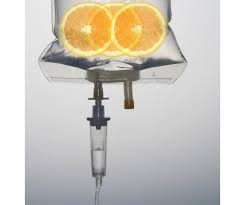
Has the Food and Drug Administration engineered a shortage of intravenous vitamin C as part of an overall attack on natural and non-toxic approaches to healing that compete with prescription drugs? An analysis by Natural Blaze would suggest that the answer is yes.
Natural Blaze claims that a critical shortage of IV bags in general followed an FDA ban on the mass production of intravenous vitamin C. The FDA limited the availability of IV-C and the pharmaceutical industry halted production of injectable vitamins and minerals, after a 60 minute story about the miraculous recovery of a swine flu patient on life support. Because of the shortage of IV-C, doctors called upon compounding pharmacies to produce it. But the FDA began to limit compounding pharmacies after injectable steroids produced by the New England Compounding Center were contaminated with a fungus that caused a deadly outbreak of meningitis. Here is an example of an entire industry being punished for the dubious practices of one compounding pharmacy.



