
Breaking News
 Shocking Email From Epstein Files Implicates Former US Ambassador, Clintons, Bidens,...
Shocking Email From Epstein Files Implicates Former US Ambassador, Clintons, Bidens,...
 Episode 490 - The 9th Annual Fake News Awards
Episode 490 - The 9th Annual Fake News Awards
 Epstein Files Dump, Gov't Shuts Down, Trump ROASTS Don Lemon...
Epstein Files Dump, Gov't Shuts Down, Trump ROASTS Don Lemon...
 SILVER MASSACRE: The Crash Reveals CME-Shanghai Coordination (Bullish Signal)
SILVER MASSACRE: The Crash Reveals CME-Shanghai Coordination (Bullish Signal)
Top Tech News
 Critical Linux Warning: 800,000 Devices Are EXPOSED
Critical Linux Warning: 800,000 Devices Are EXPOSED
 'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
 The smartphone just fired a warning shot at the camera industry.
The smartphone just fired a warning shot at the camera industry.
 A revolutionary breakthrough in dental science is changing how we fight tooth decay
A revolutionary breakthrough in dental science is changing how we fight tooth decay
 Docan Energy "Panda": 32kWh for $2,530!
Docan Energy "Panda": 32kWh for $2,530!
 Rugged phone with multi-day battery life doubles as a 1080p projector
Rugged phone with multi-day battery life doubles as a 1080p projector
 4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
 Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
 New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
 Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
Power dense zinc-manganese power unit as cheap as a car battery
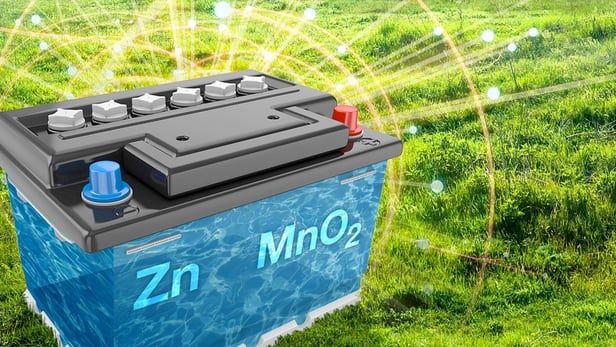
A team of scientists working on analyzing energy flows in prototype zinc-manganese batteries have stumbled upon a new way to make these power cells much more reliable, with many more recharge cycles than the humble lead-acid car battery, but costing around the same to produce. The creators claim that the new battery could become an inexpensive, ecologically-sound alternative for storing energy from renewable sources and a high-density solution for storing excess energy from the power grid.
Working at the Department of Energy's Pacific Northwest National Laboratory (PNNL), the researchers discovered a new way to approach the reliability problems of zinc-manganese batteries, that were cheap and easy to make from abundant materials, but which would fail after only a few charge cycles.
"The idea of a rechargeable zinc-manganese battery isn't new; researchers have been studying them as an inexpensive, safe alternative to lithium-ion batteries since the late 1990s," said PNNL Laboratory Fellow Jun Liu. "But these batteries usually stop working after just a few charges. Our research suggests these failures could have occurred because we failed to control chemical equilibrium in rechargeable zinc-manganese energy storage systems."



