
Breaking News
 Meet the New Porn: Even Worse than the Old Porn
Meet the New Porn: Even Worse than the Old Porn
 The unbearable pettiness of celebrity award shows
The unbearable pettiness of celebrity award shows
 Leftist Censors Cry About Censorship
Leftist Censors Cry About Censorship
 Natural solutions to hypertension: How beets, garlic and leafy greens can lower blood pressure...
Natural solutions to hypertension: How beets, garlic and leafy greens can lower blood pressure...
Top Tech News
 Critical Linux Warning: 800,000 Devices Are EXPOSED
Critical Linux Warning: 800,000 Devices Are EXPOSED
 'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
'Brave New World': IVF Company's Eugenics Tool Lets Couples Pick 'Best' Baby, Di
 The smartphone just fired a warning shot at the camera industry.
The smartphone just fired a warning shot at the camera industry.
 A revolutionary breakthrough in dental science is changing how we fight tooth decay
A revolutionary breakthrough in dental science is changing how we fight tooth decay
 Docan Energy "Panda": 32kWh for $2,530!
Docan Energy "Panda": 32kWh for $2,530!
 Rugged phone with multi-day battery life doubles as a 1080p projector
Rugged phone with multi-day battery life doubles as a 1080p projector
 4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
4 Sisters Invent Electric Tractor with Mom and Dad and it's Selling in 5 Countries
 Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
Lab–grown LIFE takes a major step forward – as scientists use AI to create a virus never seen be
 New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
New Electric 'Donut Motor' Makes 856 HP but Weighs Just 88 Pounds
 Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
Donut Lab Says It Cracked Solid-State Batteries. Experts Have Questions.
Lithium-ion battery boost could come from "caging" silicon in graphene
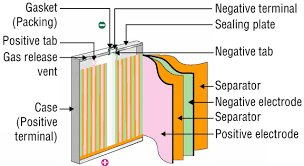
The researchers managed to remove two long-standing barriers to these improvements by putting silicon particles in graphene "cages."
To improve capacity in recent years batteries have begun to use silicon anodes, which have more capacity than the graphite conventionally used. But silicon particles also swell so much during charging that they're prone to cracking or shattering and they can also react with the battery electrolyte, forming a coating that reduces performance.
The solution from the team at Stanford and the Department of Energy's SLAC National Accelerator Laboratory is to encase each silicon particle in a "custom-fit cage" of graphene. At only one-atom thick, graphene is the thinnest, strongest form of carbon and also conducts electricity well.
The carbon cages would allow the silicon to expand and even break apart, but keep the pieces together so that they can continue to function. The graphene barrier would also block the destructive chemical reactions with the electrolyte from occurring.



